

Absence of chaperonins in cells results in cell death as demonstrated in bacterial, yeast, and mouse models ( Cheng et al., 1989 Fayet et al., 1989 Horwich et al., 1993 Fang and Cheng, 2002 Fan et al., 2020).

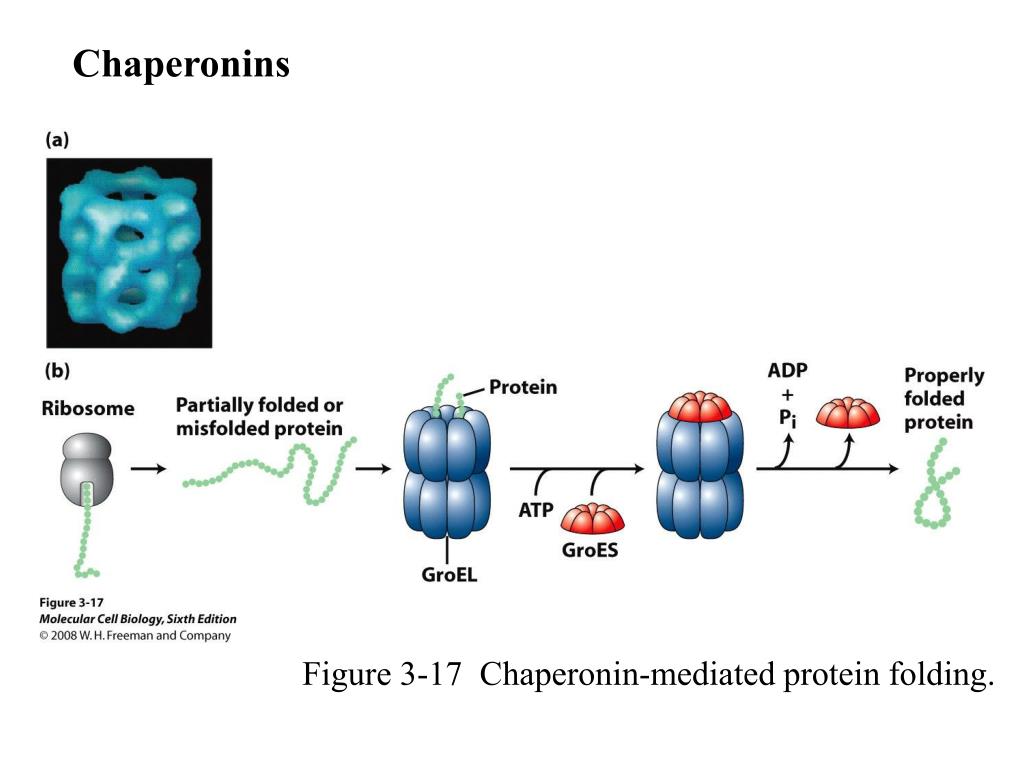
Chaperonins form macromolecular protein complexes that assist the proper folding of nascent proteins to obtain the native state and to refold misfolded proteins to prevent aggregation. Protein folding is an important aspect of cellular function and viability because the accumulation of misfolded proteins leads to the formation of insoluble aggregates that ultimately cause cell death. Here, we discuss recent studies that highlight key aspects of the mtHsp60 mechanism with a focus on some of the known disease-causing point mutations, D29G and V98I, and their effect on the protein folding reaction cycle. Although the complete protein folding mechanism of mtHsp60 is not well understood, recent work suggests that several of these mutations act by destabilizing the oligomeric stability of mtHsp60. These phenotypes are likely due to hindered energy producing pathways involved in cellular respiration resulting in ATP deprived cells. Carriers of these mutations usually develop neuropathies and paraplegias at different stages of their lives mainly characterized by leg stiffness and weakness as well as degeneration of spinal cord nerves. Individuals who harbor mtHsp60 mutations that negatively impact its folding ability display phenotypes with highly compromised muscle and neuron cells. Recently, various single-point mutations in the mtHsp60 encoding gene have been directly linked to neuropathies and paraplegias. It has been established that mtHsp60 plays a crucial role in the proper folding of mitochondrial proteins involved in ATP producing pathways. It functions as a macromolecular complex that provides client proteins an environment that favors proper folding in an ATP-dependent manner. In humans, HSPD1 encodes the mitochondrial Heat Shock Protein 60 (mtHsp60) chaperonin, which carries out essential protein folding reactions that help maintain mitochondrial and cellular homeostasis. Several neurological disorders have been linked to mutations in chaperonin genes and more specifically to the HSPD1 gene. Department of Chemistry and Biochemistry, The University of Texas at El Paso, El Paso, TX, United States.These data show that, in contrast to prevailing models, target proteins can maintain, and possibly acquire, significant native-like structure while chaperonin-bound.Alejandro Rodriguez, Daniel Von Salzen, Bianka A. We present evidence that these intermediates can be generated without the target protein leaving c-cpn. This results in the accumulation of folding intermediates which are chaperonin-bound, stable, and quasi-native in that they bind GTP nonexchangeably. Here we show that the cycling of alpha-tubulin by cytosolic chaperonin (c-cpn) can be uncoupled from the action of cofactors required to complete the folding reaction. The fact that target proteins are released from and rebind to different chaperonin molecules ("cycling") during a folding reaction suggests that chaperonins function by unfolding aberrantly folded molecules, allowing them multiple opportunities to reach the native state in bulk solution. Quasi-native chaperonin-bound intermediates in facilitated protein folding.Ĭhaperonins are known to facilitate protein folding, but their mechanism of action is not well understood.


 0 kommentar(er)
0 kommentar(er)
